Physics professor Dr Jürgen Schnack is researching new materials for future cooling technologies. He focuses on cooling at extremely low temperatures that are close to absolute zero. These are the necessary temperatures for very specialized applications. He has broadened his research on this topic by co-initiating the European doctoral network MolCal. This has already found one first candidate material with good cooling properties that could serve as a substitute for the gas helium.
Refrigerators cool our food with hydrocarbons such as propane. However, special applications, such as quantum technologies, require much lower temperatures coming close to the absolute zero of 0 kelvin (-273.15 degrees Celsius). Such temperatures are possible with the gas helium: the helium isotope 4He can cool down to 1.8 kelvin, which is -271.35 degrees Celsius. And the helium isotope 3He allows even lower temperatures ranging from 10 to 30 millikelvin. This is just what quantum computers need: it is only at such temperatures that the necessary quantum effects occur.
© Patrick Pollmeier
Starting this year, Jürgen Schnack from the Faculty of Physics supervises doctoral students in the MSCA network MolCal (Molecule-based magneto/electro/mechano-Calorics). He is working with them to find new materials that will enable future cooling technologies to work at extremely low temperatures—and thus replace helium. But why? First, helium is available only in small quantities. Whereas 4He can be extracted from natural gas as a trace element, 3He is very rare. It is produced as a waste product in nuclear power plants and can be found in very low concentrations in the Earth’s mantle. Second, ‘3He is terribly expensive,’ says Schnack.
New cooling technology using crystallites
Specifically, the physicist and his team are looking for molecule-based caloric solid-state materials. These can be crystals or powders made of small crystallites. Molecule-based means that the solids consist of molecular units that are arranged regularly. Caloric means that the materials change temperature when exposed to certain stimuli—for example, changes in pressure and expansion—which, in the latter case, means that the materials are mechanocaloric. However, there are also electrocaloric and magnetocaloric materials that react when an electric or magnetic field is switched on and off. The magnetocaloric effect is Schnack’s speciality and the main focus at MolCal.
The project is a European one. Research teams from e.g. Spain, Greece, Scotland, and Bielefeld are working together on an interdisciplinary basis. The division of labour is as follows: chemists in Crete and Edinburgh synthesize new materials that are then analysed by experimental physicists in Zaragoza. Schnack’s group takes care of the modelling: ‘We’re responsible for the theory. We have nothing to do with experiments and substances, we just produce quantum mechanical models.’ With these models, something crucial is taken into account: the interactions between the magnetic atoms or ions. ‘Our models show why a material possesses certain magnetic properties. Otherwise, there would only be observations from the experiments. We provide the explanations. To really understand a material, you need our calculations.’
Spins located at the nodes
These calculations are a kind of simplification of the complicated material structure consisting of thousands of particles. Everything that has nothing to do with magnetism is gradually dropped from the calculations until all that is left is the magnetically active ions. These are then also reduced to a single property: their spin. Spin is the intrinsic angular momentum of electrons that causes the magnetic moment of atoms. ‘We end up with a small, clear geometric structure: our model. In this model, the spins are located at the nodes, and connecting lines represent the interactions between the spins.’
Mathematically, such models are known as Hamiltonian matrices. However, these pose a problem: how to save and store them. Storing larger molecules would require over 100 terabytes of RAM. ‘There are no computers with this kind of RAM. That’s why we apply a clever mathematical method. We determine the magnetic properties hidden in the matrix approximately, without storing the matrix even once. That is our expertise. We are the only group in the world that has mastered these approximation equations for such large systems.’

© Patrick Pollmeier
New material cools down to 0.4 kelvin
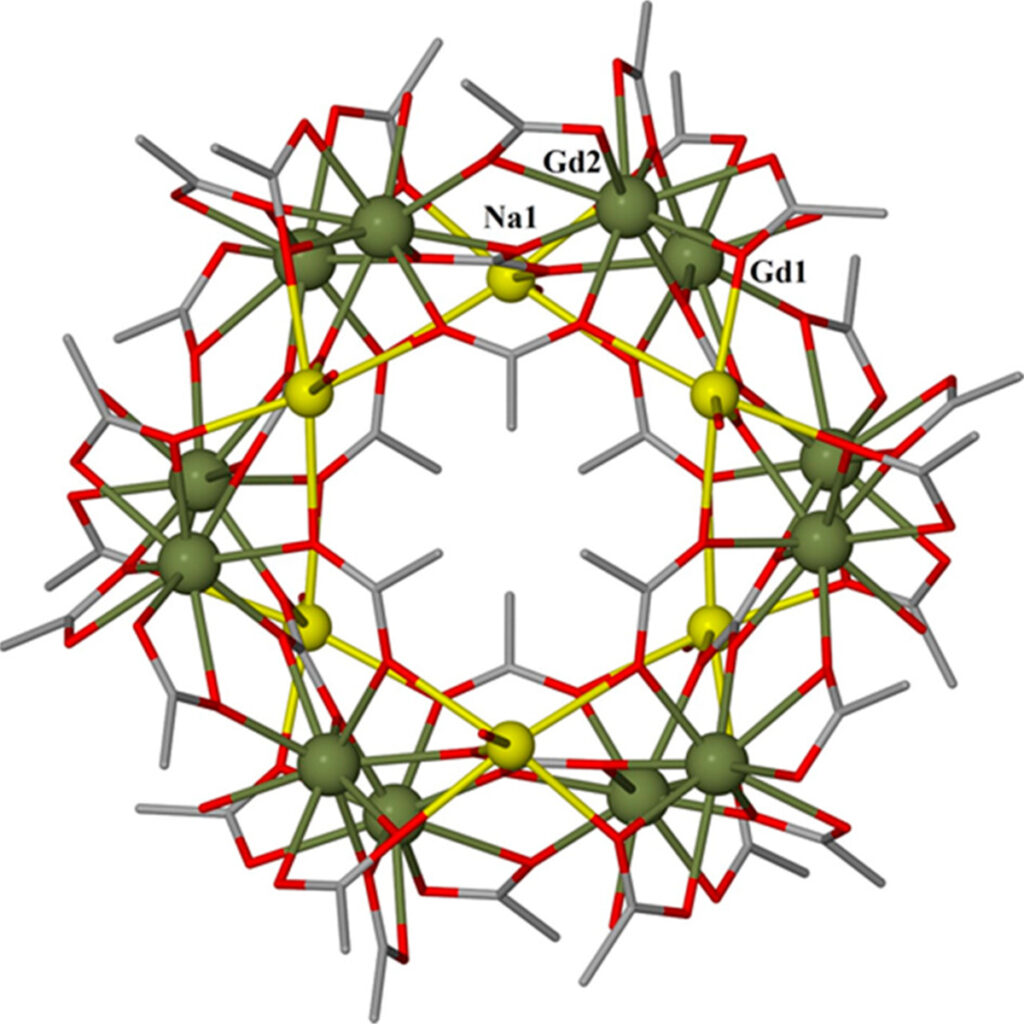
MolCal has also already found one cooling candidate. This material has the abbreviated chemical formula Gd12 Na6. Hence, the main components of this pale yellow crystallite are gadolinium and sodium. The sodium serves only as a molecular scaffold: it is the gadolinium that is decisive in magnetocaloric terms. ‘Gadolinium has a particularly large magnetic moment and therefore it often has a large magnetocaloric effect as well,’ explains Schnack. Tests confirmed this: the material functioned very well in the temperature range between 0.4 and 2 kelvin. This means that it could be used in space missions, for example, in the LiteBIRD research project. This project is using satellites to study cosmic background radiation. The cooling temperature required for the telescopes is exactly in the range covered by Gd12 Na6.
However, Gd12 Na6 cannot replace helium completely, and especially not 3He. Moreover, that isn’t what Schnack intends. ‘The extremely low temperatures down to the millikelvin range cannot be covered by a single solid-state material; only 3He can do that. It’s a cooling miracle.’ Hence, future materials will have to share the task. Gd12 Na6 would cool in the 2 to 0.4 kelvin range. And above and below that, other materials would take over that have yet to be discovered. Schnack’s modelling is also helpful here: understanding the magnetic interactions in known materials makes it possible to narrow the search for new ones.
© Thomais G Tziotzi et al., CC-BY 4.0 *
Efficiency is also important
A second aspect that plays a role in future cooling materials is the number of cooling cycles. The amount of heat transported into the environment per process cycle should be as large as possible. This is because a cooling machine has friction losses, and these are minimized when there are fewer cycles. The factor directly related to this is called isothermal entropy change: if this is large, the amount of heat transferred per cycle is also large. Here too, gadolinium has promising properties.
Image of the Gd-Na molecule taken from this study:
Thomais G. Tziotzi, David Gracia, Scott J. Dalgarno, Jürgen Schnack, Marco Evangelisti, Euan K. Brechin, Constantinos J. Milios: A {Gd12Na6} Molecular Quadruple-Wheel with a Record Magnetocaloric Effect at Low Magnetic Fields and Temperatures. Journal of the American Chemical Society, https://doi.org/10.1021/jacs.3c01610, published on 3 April 2023.