Scientists from Germany, Spain and the USA have investigated one of the oldest questions in biology: They have investigated how cells of marine sponges assemble to form a multicellular organism. Large molecules known as Aggregation Factors (AF) are responsible for this – they act like a natural glue between the cells. The researchers wanted to understand how AFs are structured and whether they are similar to the bonding mechanisms of other animals. The results of the study, in which biophysicist Professor Dr Dario Anselmetti from Bielefeld University was involved, were published in the renowned scientific journal Proceedings of the National Academy of Sciences (PNAS).
Why are sponges a good model for studying cell adhesion?
Dario Anselmetti: Sponges in general and the sponge Clathria prolifera are among the oldest fossilised and multicellular organisms. The transition from unicellular to multicellular organisms was a decisive step in evolution and the basis for our own existence. One of the unanswered questions in biology is what causes and mechanisms made this change possible. This process began around 500 million years ago and heralded the ‘Cambrian explosion of life’, a phase of rapid biodiversity.

© Michael Adamski
So you are dealing with one of the oldest questions in biology.
Dario Anselmetti: Exactly. As early as 1907, the Wilson experiment provided the first indications of species-specific cell adhesion. Sponges of different colours were treated in water in such a way that their cells separated. When they reassembled, the red cells formed a red sponge and the yellow cells formed a yellow sponge. This was the first proof of the existence of species-selective cell adhesion molecules, which we call allorecognition.
You have been researching this topic for decades. What did you think in the past about how cells adhere to each other – and what do you know today?
Dario Anselmetti: Since 1994, my colleague Xavier Fernàndez-Busquets from the Institute of Bioengeneering of Catalonia in Barcelona and I have been investigating the molecular bonds between sponge cells and the AF molecules involved. For this purpose, we glued the isolated AF molecules to a mechanical probe of an atomic force microscope (AFM) and brought them into contact with a counterpart with the second aggregation factor. We then pulled them apart to measure the binding between individual molecular binding partners. At that time, however, we did not yet know exactly how the bond worked. It was clear that the aggregation factor of sponges has a sunray-like structure – like a ring from which rays emanate. We were able to visualise this with the special AFM microscope. It was assumed that the ring was located on the cell and the rays, which look like tentacles, would connect during adhesion. Today we know: The tentacles lie on the surface of the cell and the ring-shaped part interacts with the surface of the cell. A small sugar molecule called g-200 is located there, which ensures that the cells recognise each other and connect.
You have now investigated the molecular composition of the Aggregation Factor (AF) in more detail. What did you find out?
Dario Anselmetti: Our study has shown that the AF is more similar to other animal cell compounds than previously thought. It was assumed that the AF is a unique system that only exists in sponges. But our analyses show that some of its components and parts have strong similarities with proteins that regulate cell connections in other animals today, or control other processes such as blood clotting or immune recognition.
Does this mean that the mechanism of cell adhesion in sponges and other animals has a common evolutionary basis?
Dario Anselmetti: That’s exactly what we asked ourselves – whether there are still similarities in the molecular structures of sponges today with those of animals or humans. Our results suggest that there is an ancient toolbox of protein domains that the first multicellular animals used to connect cells with each other and to distinguish between ‘self’ and ‘other’. These mechanisms have developed differently over the course of evolution, but the basic principles of nature appear to be conserved.
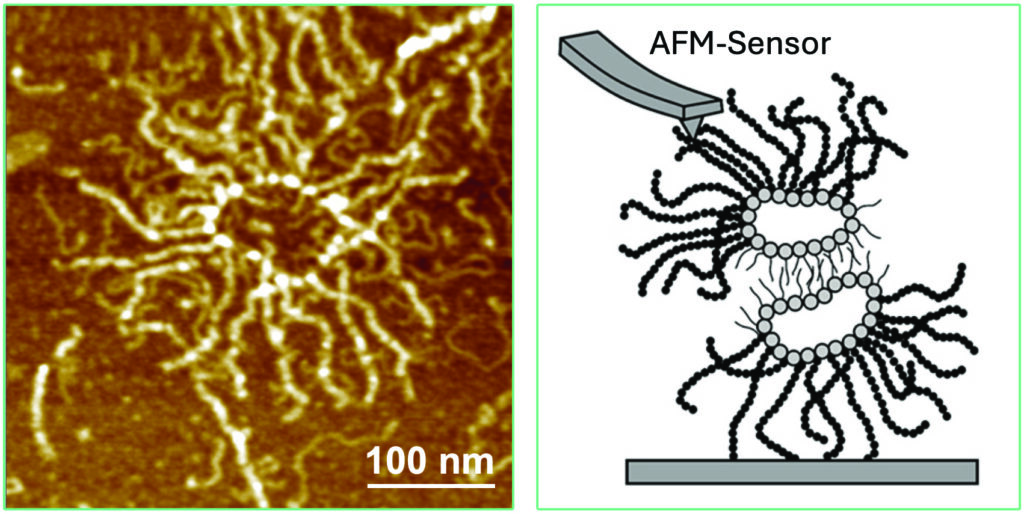
© Dario Anselmetti/Xavier Fernàndez-Busquets
What significance does this principle have for living beings?
Dario Anselmetti: This recognition of foreign and own is a central element of growth. This happens through many billions of interactions between molecules that find their binding partner in the right place at the right time. Cell-cell recognition takes place everywhere, for example in embryogenesis. Our survival also depends crucially on our immune system functioning and being able to recognise and fight foreign invaders. It is partly inherited from our parents but can also train itself through external influences to which the body can then react.
How did you go about analysing the aggregation factor?
Dario Anselmetti: Proteomics, i.e. analysing all the proteins present, was crucial in order to find out which proteins are actually part of the AF. We carried out a mass spectrometry analysis to determine the protein composition in different AF samples. We then matched this experimental data with our computerised AlphaFold simulation analyses to see if the predicted structures were present in the real AF proteins. The AlphaFold technology uses artificial intelligence to predict the three-dimensional structure of a protein from its amino acid sequence and was awarded the Nobel Prize in Chemistry in 2024. It analyses known protein structures and searches for patterns that indicate a certain folding.
In your decades of research, the methods and systems have evolved. How does it feel to have been researching a topic for so long?
Dario Anselmetti: Of course you realise how old you are by now (laughs). But it’s also incredibly satisfying. Especially because we worked with complex, unknown molecules instead of taking the quicker route with simpler, known molecules. The interdisciplinary collaboration with biologists, physicians and physicists and the investigation of the same scientific topic from different perspectives has enriched the project enormously. It is exciting to tell the story of our discoveries, even if it includes many dead ends and setbacks – as research goes. What is particularly valuable is not only that this topic has been with me for over 30 years, but also that I have learnt from nature and its evolutionary adaptation strategies, which has led to many new biomedical insights – a true example of sustainable research.

© James M. Davidson/stock.adobe.com
Transparency notice: This translation was created with machine assistance and subsequently edited.